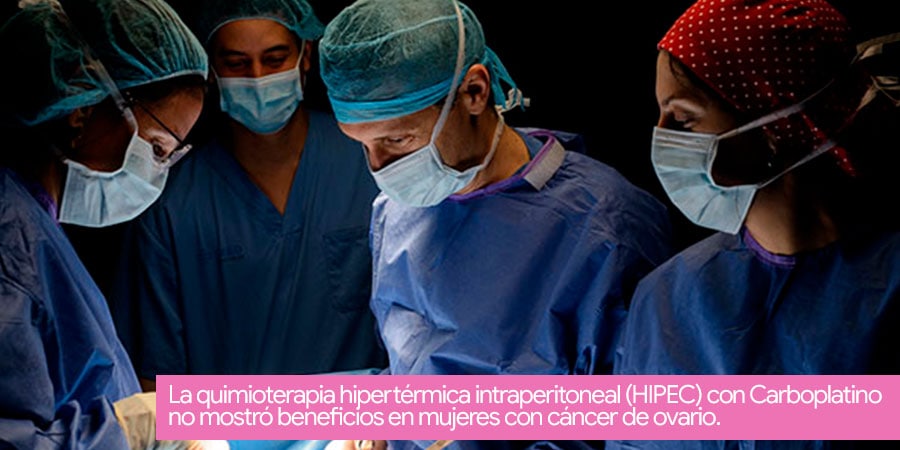
Hyperthermic Intraperitoneal Chemotherapy (HIPEC) with Carboplatin did not show benefits in women with ovarian cancer.
Although hyperthermic intraperitoneal chemotherapy (HIPEC) seemed to be well tolerated in patients with recurrent platinum-resistant ovarian cancer (a type of chemotherapy), the regimen does not seem to be effective during a secondary cytoreductive surgery (in case of a first recurrence), according to the results of a phase 2 study (NCT01767675) published in the Journal of Clinical Oncology.
At 24 months, 8 patients (16,3%; one sided CI 90%, 9,7% to 100%) in the HIPEC group and 12 patients (24,5%; one sided CI 90%, 16,5% to 100%) in the standar treatment arm did not have progression or death. The mean progression-free survival (PFS) was 14,3 months (CI of 95%, 12-16) and global survival (GS) was 55,2 months (CI of 95%, 50,3-78,0) for the entire cohort.
Additionally, researchers found that patients randomized to receive HIPEC had a mean PFS of 12,3 months compared to 15,7 months in the standard treatment arm (HR, 1,54; CI of 95%, 1-2,37). In both the HIPEC and the standard treatment arms, the GS was 52,5 months and 59,7 months respectively (HR, 1,39; CI of 95%, 0,73-2,67). In a posthoc analysis, non of the groups showed a recurrence pattern. Also, the researchers found that patients with a BRCA mutation did not get any oncologic benefit from the HIPEC regimen.
The goal of the study was to evaluate if the combination of HIPEC with carboplatin could improve the results in patients with recurrent ovarian cancer who underwent a secondary cytoreductive surgery for the first recurrence of a platinum-sensitive disease. The investigators were hoping that HIPEC would facilitate the destruction of cancerous cells.
The study included 99 patients who were randomized to receive either secondary cytoreductive surgery followed by 5 cycles of HIPEC and carboplatin at a dose of 800 mg / m2 or secondary cytoreductive surgery followed by 6 cycles of carboplatin-based chemotherapy. After receiving HIPEC, patients were administered 3000 ml of saline solution at 41 ° C to 43 ° C that circulated through the abdomen after surgery. Carboplatin was added to the patients’ catheter perfusion system.
The main outcome of the study was the proportion of patients without evidence of disease progression at 24 months, and the secondary outcome were postoperative morbidity, GS pharmacokinetics, and being able to complete postoperative chemotherapy at 30 days.
To enroll, patients had to be 21 one years or older, and have the first high grade epithelial ovarian cancer recurrence 6-30 months after finishing first-line platin-based chemotherapy. Patients also had to be eligible for secondary cytoreductive surgery and previous treatment with chemotherapy for recurrent disease was not allowed.
Before their inclusion in the study, the mean platinum-free interval for the entire cohort was 16 months. After enrolment, 69% of patients had a platinum-free interval of 12 to 30 months. In total, 82% (n = 40) of the HIPEC patients had undergone a successful complete macroscopic resection, along with 94% (n = 46) of the standard treatment group (p = 0,12). Fifty patients (51%) underwent an intestinal resection, although significantly fewer were performed in the HIPEC group. Additionally, the mean duration of the surgery was significantly higher in patients randomized to the HIPEC arm (474 minutes against 292 minutos; p <0,001).
In the 30-day follow-up, there were no mortality reports. Twelve patients from the HIPEC group and 10 from the standard group (P = 0.81) experienced grade 3 or higher adverse effects (AE). Some of the most common AE in the HIPEC and standard treatment cohorts included anemia (14,3% against 16,3%), urinary tract infection (8,2% against 0%), and ileus (4,1% against 2%). In the last follow-up, 82 patients had progressed, while 37 had died.
More studies are definitely needed to address the best way of incorporating HIPEC in the treatment of women with recurrent ovarian cancer.
![[:es]Telemedicina Médico remoto en España NOTICIA[:]](https://drlucasminig.com/en/wp-content/uploads/telemedicina-medico-remoto-doctor-espana-lucas-minig.jpg)
![[:es]La quimioterapia hipertérmica intraperitoneal (HIPEC), no es útil en mujeres con cáncer de ovario[:]](https://drlucasminig.com/en/wp-content/uploads/quimioterapia-hipertermica-intraperitoneal-no-util-cancer-de-ovario.jpg)
![[:es]Vacuna Contra VPH reduce cáncer de útero[:]](https://drlucasminig.com/en/wp-content/uploads/vacuna-contra-vph-noticias-dr-lucasminig.jpg)
![[:es]Investigan cómo prevenir el cáncer de ovario. Doctor Lucas Minig[:]](https://drlucasminig.com/en/wp-content/uploads/prevenir-cancer-de-ovario-mujeres-con-alto-riesgo-drlucasminig-investigacion.jpg)
